Researchers Report Progress On A Solid-State Lithium-Air Battery With High Energy Density
Researchers at the Illinois Institute of Technology, University of Illinois-Chicago, and Argonne National Labs, have succeeded in producing a practical demonstration of a lithium-air battery that achieves an energy density of 685 Wh/kg at room temperature. Furthermore, they claim their new battery will be inexpensive to produce and safer than a conventional lithium-ion battery because it is solid-state, meaning it contains no liquids that can leak or catch fire. Here is the abstract to a report published February 2 in the journal Science (paywall).
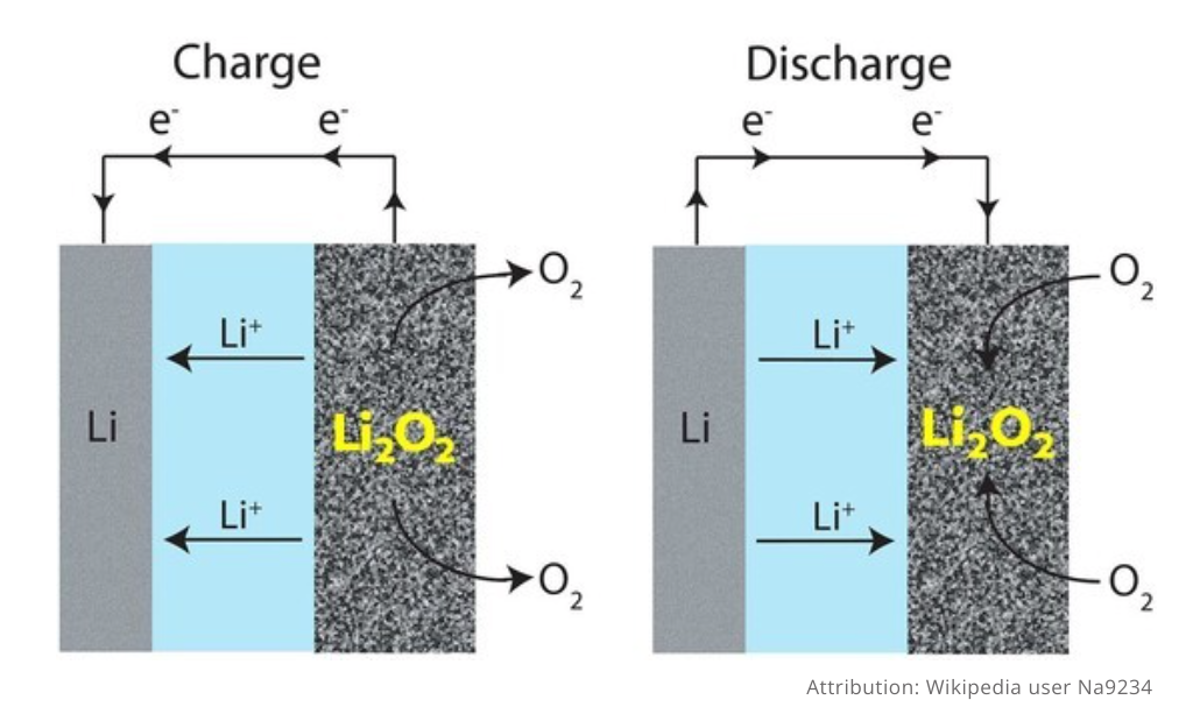
A lithium-air battery based on lithium oxide (Li2O) formation can theoretically deliver an energy density that is comparable to that of gasoline. Lithium oxide formation involves a four electron reaction that is more difficult to achieve than the one and two electron reaction processes that result in lithium superoxide (LiO2) and lithium peroxide (Li2O2), respectively.
By using a composite polymer electrolyte based on Li10GeP2S12 nanoparticles embedded in a modified polyethylene oxide polymer matrix, we found that Li2O is the main product in a room temperature solid state lithium air battery. The battery is rechargeable for 1000 cycles with a low polarization gap and can operate at high rates. The four electron reaction is enabled by a mixed ion electron conducting discharge product and its interface with air.
One of the 12 researchers involved in the lithium-air battery research is Mohammad Asadi, an assistant professor of chemical engineering at Illinois Institute of Technology. In a press release, IIT says the battery design Asadi and his colleagues created has the potential to store one kilowatt-hour of electricity per kilogram — four times greater than current lithium-ion battery technology. That would represent a transformation for electric transportation, especially heavy duty vehicles such as airplanes, trains, and submarines.
Asadi started out to make a battery with a solid electrolyte, which provides safety and energy benefits compared to liquid electrolyte batteries. He chose a mix of polymer and ceramic, which are the two most common solid electrolytes, but both have drawbacks. By combining them, Asadi found he could take advantage of ceramic’s high ionic conductivity as well as the high stability and high interfacial connection of the polymer.
The result allows for the critical reversible reaction that enables the battery to function — lithium dioxide formation and decomposition — to occur at high rates at room temperature, the first time this has been possible in a lithium-air battery.
“We found that that solid state electrolyte contributes around 75 percent of the total energy density. That tells us there is a lot of room for improvement because we believe we can minimize that thickness without compromising performance and that would allow us to achieve a very, very high energy density,” says Asadi.
He says he plans to work with industry partners as he moves toward optimizing the battery design and engineering it for manufacturing. “The technology is a breakthrough and it has opened up a big window of possibility for taking these technologies to the market,” Asadi says.
A Cheap But Effective Solid-State Electrolyte
One of the main contributions by the authors is that they developed a lightweight polymer-ceramic composite that conducts Li+ ions about 15x better at room temperature than other solid materials that have been tried up to now, says The Daily Kos. Others have come up with very good Li+ conductors for lithium-air batteries, but they were made of molten salts that are liquid and heavy. They also need high temperatures to work effectively, so they are neither safe nor inexpensive.
But there’s another key achievement here, The Daily Kos points out. In previous lithium-air batteries, Li2O2 transfers two electrons from lithium for every oxygen, but in this new prototype, the chemical reaction looks like this:
4 Li+ + 4 e- + O2 → 2 Li2O (4 e- per O2)
One key problem with using Li2O at room temperature is that the transition state — Li2O2 –would rather donate its electrons back to oxygen. How do the researchers keep that from happening when by definition there is lots of oxygen available? They freely admit they don’t fully understand the process yet, but it probably goes something like this, according to The Daily Kos.
First, the electrolyte is such a good Li+ conductor it allows excess Li+ to move rapidly whereas before the Li+ just couldn’t travel through the electrolyte fast enough to keep up.
Second, the products that form first, LiO2 and Li2O2, seem to form a coating over the surface of the catalyst material that still conducts ions but won’t let oxygen through so the Li2O2 can be further converted to Li2O without being hindered by oxygen. LiO2 and Li2O2 form for about 15 minutes of battery discharge. After that, it’s all Li2O until the battery runs out of juice.
One of the biggest advantages of the catalyst the researchers designed for the cathode is that it is made of molybdenum phosphide, which is abundant and inexpensive. So they not only made a great solid state Li+ conductor, they also made an inexpensive catalyst that is good at promoting forward and backward reactions with oxygen so the system is rechargeable. They have tested their new lithium-air battery through 1,000 cycles and noted very little dropoff in performance.
The upshot of all of this is that we may have the basis for super-efficient car batteries and for storage of renewable energy — all because of a material that lithium ions like to zoom through at room temperature.
The Takeaway
As with all news of battery advancements in the laboratory, we should be skeptical until more testing has been done and more data is available. Typically, the path from laboratory to production is five years long or more. That being said, just the thought of a battery that has an energy density approaching that of gasoline is cause for celebration. If true, that would move the “electrify everything” movement one giant step forward. It’s enough to make even the most cynical among us just a tiny bit excited about lithium-air batteries.
Have a tip for CleanTechnica? Want to advertise? Want to suggest a guest for our CleanTech Talk podcast? Contact us here.
Latest CleanTechnica.TV Video

CleanTechnica uses affiliate links. See our policy here.