Manganese Cathodes Could Boost Lithium-ion Batteries
Support CleanTechnica's work through a Substack subscription or on Stripe.
Or support our Kickstarter campaign!

Manganese is earth-abundant and cheap. A new process could help make it a contender to replace nickel and cobalt in batteries.
Rechargeable lithium-ion batteries are growing in adoption, used in devices like smartphones and laptops, electric vehicles, and energy storage systems. But supplies of nickel and cobalt commonly used in the cathodes of these batteries are limited. New research led by the Department of Energy’s Lawrence Berkeley National Laboratory (Berkeley Lab) opens up a potential low-cost, safe alternative in manganese, the fifth most abundant metal in the Earth’s crust.
Researchers showed that manganese can be effectively used in emerging cathode materials called disordered rock salts, or DRX. Previous research suggested that to perform well, DRX materials had to be ground down to nanosized particles in an energy-intensive process. But the new study found that manganese-based cathodes can actually excel with particles that are about 1000 times larger than expected. The work was published Sept. 19 in the journal Nature Nanotechnology.
“There are many ways to generate power with renewable energy, but the importance lies in how you store it,” said Han-Ming Hau, who researches battery technology as part of Berkeley Lab’s Ceder Group and is a PhD student at UC Berkeley. “By applying our new approach, we can use a material that is both earth-abundant and low-cost, and that takes less energy and time to produce than some commercialized Li-ion battery cathode materials. And it can store as much energy and work just as well.”
The researchers used a novel two-day process that first removes lithium ions from the cathode material and then heats it at low temperatures (about 200 degrees Celsius). This contrasts with the existing process for manganese-based DRX materials, which takes more than three weeks of treatment.
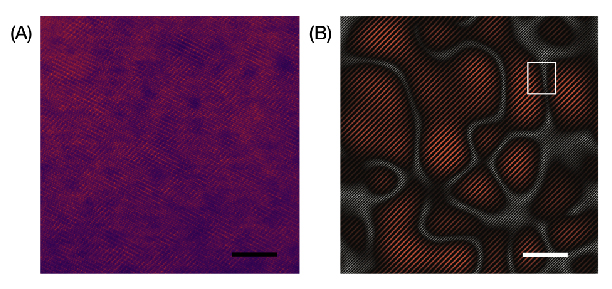
Researchers used state-of-the-art electron microscopes to capture atomic-scale pictures of the manganese-based material in action. They found that after applying their process, the material formed a nanoscale semi-ordered structure that actually enhanced the battery performance, allowing it to densely store and deliver energy.
The team also used different techniques with X-rays to study how battery cycling causes chemical changes to manganese and oxygen at the macroscopic level. By studying how the manganese material behaves at different scales, the team opens up different methods for making manganese-based cathodes and insights into nano-engineering future battery materials.
“We now have a better understanding of the unique nanostructure of the material,” Hau said, “and a synthesis process to cause this ‘phase change’ in the material that improves its electrochemical performance. It’s an important step that pushes this material closer to battery applications in the real world.”
This research used resources at three DOE Office of Science user facilities: the Advanced Light Source and Molecular Foundry (National Center for Electron Microscopy) at Berkeley Lab, and the National Synchrotron Light Source II at Brookhaven National Laboratory. The work was supported by DOE’s Office of Energy Efficiency and Renewable Energy and Office of Science.
Courtesy of Lawrence Berkeley National Laboratory (Berkeley Lab). By Lauren Biron.
Support CleanTechnica via Kickstarter

Sign up for CleanTechnica's Weekly Substack for Zach and Scott's in-depth analyses and high level summaries, sign up for our daily newsletter, and follow us on Google News!
Have a tip for CleanTechnica? Want to advertise? Want to suggest a guest for our CleanTech Talk podcast? Contact us here.
Sign up for our daily newsletter for 15 new cleantech stories a day. Or sign up for our weekly one on top stories of the week if daily is too frequent.
CleanTechnica uses affiliate links. See our policy here.
CleanTechnica's Comment Policy

